Application
How is titanium dioxide manufactured?
The scientific name of titanium dioxide is titanium dioxide, the molecular formula is TiO2, and the relative molecular mass is 79.90. It is an inert pigment and is considered to be the best white pigment in the world.
It has two structures: rutile type (Rtile R type) and anatase type (Anatase A type). The rutile crystal structure is compact, relatively stable, and has low optical activity, so it has good weather resistance, and has high hiding power and color reduction power. .
How is titanium dioxide manufactured?
The main manufacturing methods of titanium dioxide are sulfuric acid method and chlorination method. Among them, about 56% of titanium dioxide is the product of chlorination method, and more than 70% of this product is produced by large titanium dioxide factories such as DuPont in the United States, and other countries Including China's titanium dioxide factories are still dominated by the sulfuric acid method.
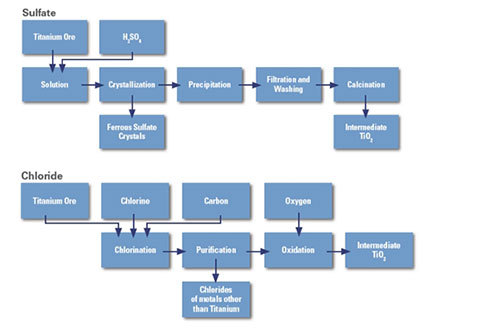
Sulfuric acid method
Use concentrate titanium or acid-soluble titanium slag to react with sulfuric acid for acid hydrolysis to obtain titanyl sulfate solution, which is hydrolyzed to obtain metatitanic acid precipitation; then enter the rotary kiln for calcination to produce titanium dioxide. The sulfuric acid method can produce both anatase titanium dioxide and rutile titanium dioxide.
Chlorination method
Use titanium-containing raw materials, react with chlorine gas to generate titanium tetrachloride with chlorinated high-titanium slag, artificial rutile, or natural rutile, which is purified by rectification, and then undergoes gas-phase oxidation; after rapid cooling, titanium dioxide is obtained through gas-solid separation.